- All
- 2026
- 2025
- 2024
- 2023
- 2022
- 2021
- 2020
- 2019
- 2018
- 2017
- 2016
- 2015

AmoyDx® Pan Lung Cancer PCR Panel Approved in Japan as a Companion Diagnostic for LUMAKRAS® (Sotorasib)
Riken Genesis Co., Ltd., Amoy Diagnostics Co., Ltd., (“AmoyDx”) and Precision Medicine Asia Co., Ltd. (“PREMIA”) today announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) has approved the AmoyDx® Pan Lung Cancer PCR Panel (the “AmoyD

AmoyDx and BeiGene Announce Strategic Cooperation on HER2 Companion Diagnostics
Amoy Diagnostics (300685.SZ) and BeiGene (NASDAQ: BGNE; HKEX: 06160; SSE: 688235) announced a strategic partnership in the field of companion diagnostics.

Amoy Diagnostics and Roche Pharma China Jointly Announce Strategic Partnership
The 2nd Roche Pan-Tumor Precision Medicine Innovation Forum was officially held at the Shanghai Roche Pharmaceuticals Campus, where participants had discussions on recent progresses in this field.

AmoyDx® Master Panel--Study Reveals Clinically Relevant Intertumoral Heterogeneity of NSCLCs Driven by MET Exon 14 Skipping Mutation
Vienna – Aug. 7th, 2022. – A study presented at the IASLC World Conference on Lung Cancer 2022 in Vienna disclosed the clinically relevant intertumoral heterogeneity of non-small cell lung cancer (NSCLC) driven by MET exon 14 skipping (METex14 skipping).

MHLW Approves AmoyDx® Pan Lung Cancer PCR Panel as Companion Diagnostic for Rozlytrek® (Entrectinib) in Japan
TOKYO and XIAMEN, August 9, 2022 -- Riken Genesis Co., Ltd., Amoy Diagnostics Co., Ltd., (“AmoyDx”) and Precision Medicine Asia Co., Ltd. (“PREMIA”) today announced that the AmoyDx® Pan Lung Cancer PCR Panel (the “AmoyDx PLC Panel”) had been approved by t

Filing Notice for a Manufacturing and Marketing Application for AmoyDx® Pan Lung Cancer PCR Panel as a Companion Diagnostic for a Drug Targeting RET fusion-Positive Non-Small Cell Lung Cancer
TOKYO and XIAMEN, April 20, 2022 -- Riken Genesis Co., Ltd., (“Rigen Genesis”), Amoy Diagnostics Co., Ltd., (“AmoyDx”) and Precision Medicine Asia Co., Ltd. (“PREMIA”) today announced that in April 2022, AmoyDx® Pan Lung Cancer PCR Panel (the “

AmoyDx Enters into Master Collaboration Agreement with AstraZeneca for Multiple Companion Diagnostics Programs in China, EU and Japan
April 18, 2022 Shanghai, China——Amoy Diagnostics Co., Ltd (AmoyDx), a China based innovative molecular diagnostics company (SZSE: 300685), today announces that it has entered into a Master Collaboration Agreement (Agreement) with AstraZeneca (LSE/STO/Nasd

Beckman Coulter Life Sciences and Amoy Diagnostics Sign Application Development Agreement for the Biomek Ngenius Next Generation Library Prep System
Xiamen, China April 13, 2022 - Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685), an innovative commercial-stage in vitro diagnostic (IVD) company, and Beckman Coulter Life Sciences, a global leader in life sciences lab automation and innovation, announce

AmoyDx Signs Strategic Cooperation Agreement with HUTCHMED in China
Xiamen, China - Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685), an innovative commercial-stage in vitro diagnostic (IVD) company, and HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13), an innovative, commercial-stage, biopharmaceutical co
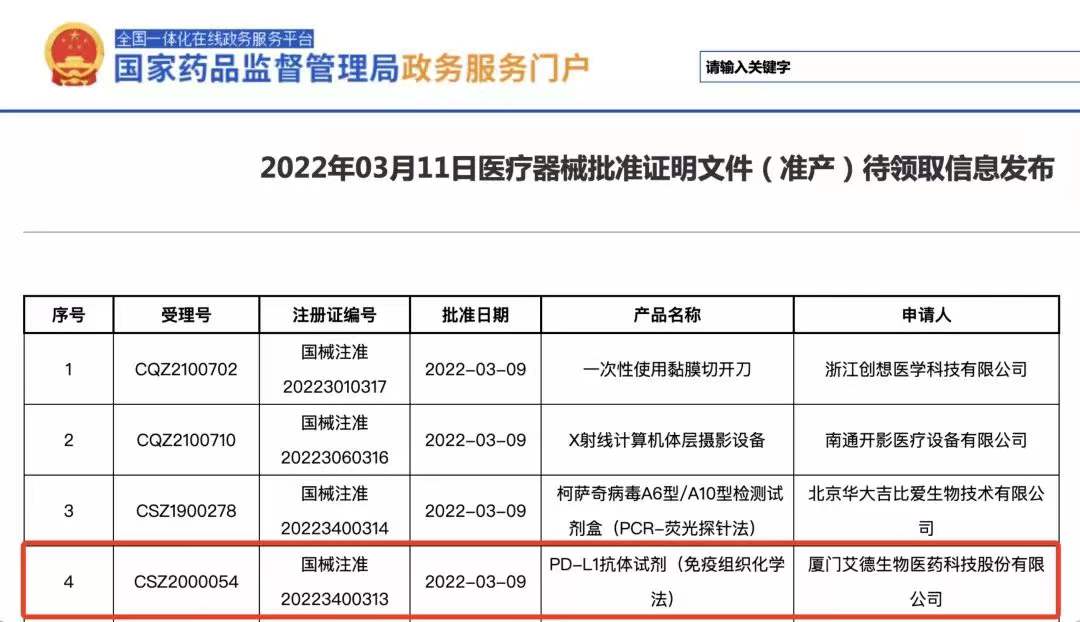
China's NMPA Granted Approval to AmoyDx® PD-L1 Expression Detection Kit (IHC)
Xiamen, China, March 15, 2022 - Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685), an innovative commercial-stage in vitro diagnostic (IVD) company today announced that China’s National Medical Products Administration (NMPA) recently granted Approval to A

AmoyDx Collaborates with Pierre Fabre to Develop Companion Diagnostics for China Market
Xiamen (China), Castres (France), February 23rd, 2022 – Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE:300685), and the French pharmaceutical group Pierre Fabre announced that the companies have entered into a collaboration to develop Companion Diagnostic (CDx)

Notice Concerning Application for Partial Revision of Manufacturing and Marketing Approval of Companion Diagnostic Reagent for the AmoyDx® Pan Lung Cancer PCR Panel
TOKYO and XIAMEN, February 24, 2022 -- Riken Genesis Co., Ltd., Amoy Diagnostics Co., Ltd., (“AmoyDx”) and Precision Medicine Asia Co., Ltd. (“PREMIA”) today announced that an application for partial revision of manufacturing and marketing approval for Am

AmoyDx Announces Its PLC Panel Adopted as Companion Diagnostic for Brigatinib in Japan
Xiamen, China, January 29, 2022 - Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685), an innovative commercial-stage in vitro diagnostic (IVD) company today announced that the AmoyDx Pan Lung Cancer PCR Panel (the “PLC Panel”) has been adopted by Takeda Ph

AmoyDx and PREMIA Announce the Commercial Launch of the AmoyDx® Pan Lung Cancer PCR Panel in Japan
Xiamen and Hong Kong, January 14, 2022 -- Amoy Diagnostics Co., Ltd., (“AmoyDx”) and PREMIA Holdings (HK) Limited (“PREMIA”) today announced the commercial launch of the AmoyDx® Pan Lung Cancer PCR Panel (the “PLC Panel”) in Japan as a reimbursed companio

Amoy Diagnostics and Janssen China Sign MoU on Strategic Collaboration
Xiamen China, December 9, 2021 – Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685) recently signed a Memorandum of Understanding (MoU) on strategic collaboration with The Janssen Pharmaceutical Companies of Johnson & Johnson. In this cooperation, both par

AmoyDx Collaborates with Amgen to Develop Companion Diagnostics for Japan Market
Xiamen, China, Aug 19, 2021 - Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685), an innovative commercial-stage in vitro diagnostic (IVD) company, and Amgen Inc. (NASDAQ: AMGN), announced today that the companies have entered into a strategic collaboratio

AmoyDx® Pan Lung Cancer PCR Panel Receives MHLW Approval as Companion Diagnostic for TEPMETKO® (tepotinib)
TOKYO and XIAMEN, August 17, 2021 (GLOBE NEWSWIRE) -- Riken Genesis Co., Ltd., Amoy Diagnostics Co., Ltd. (“AmoyDx”), and Precision Medicine Asia Co., Ltd. (“PREMIA”) today announced that the Ministry of Health, Labour and Welfare (MHLW) has approved the

AmoyDx® Pan Lung Cancer PCR Panel Receives MHLW Approval as Companion Diagnostic for 9 Targeted Therapies for Use in Patients with Advanced Non-Small Cell Lung Cancer
TOKYO and XIAMEN, June 30, 2021 (GLOBE NEWSWIRE) -- Riken Genesis Co., Ltd., Amoy Diagnostics Co., Ltd., (“AmoyDx”) and Precision Medicine Asia Co., Ltd. (“PREMIA”) today announced that the AmoyDx® Pan Lung Cancer PCR Panel (the “AmoyDx® PLC Panel”), an i
