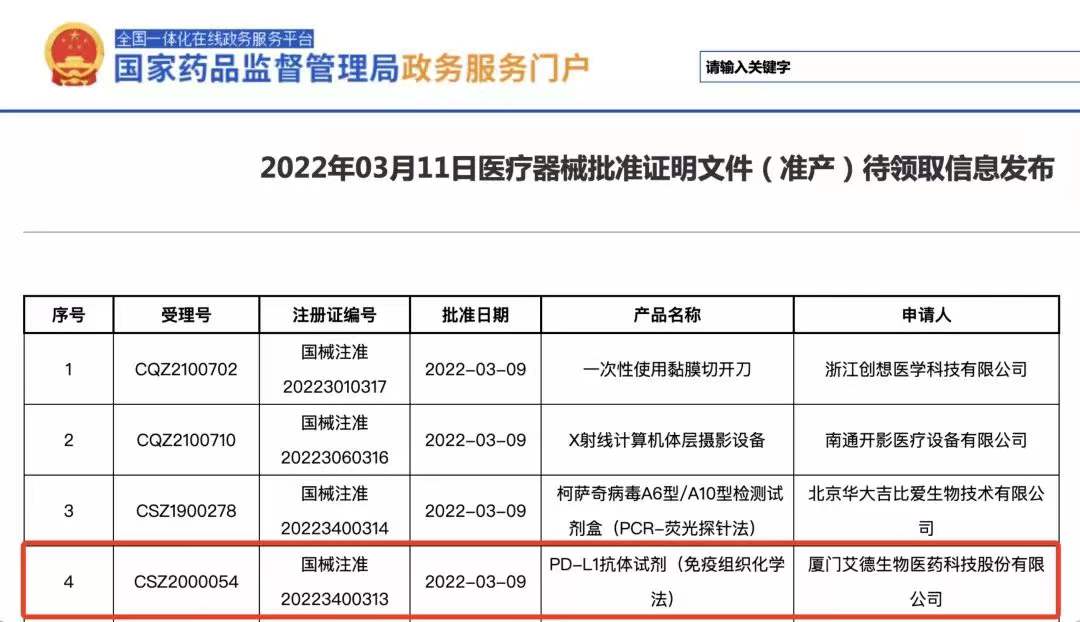
Xiamen, China, March 15, 2022 - Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685), an innovative commercial-stage in vitro diagnostic (IVD) company today announced that China’s National Medical Products Administration (NMPA) recently granted Approval to AmoyDx® PD-L1 Expression Detection Kit (IHC) for guiding the first-line treatment of Pembrolizumab in non-small cell lung cancer patients. This builds on AmoyDx’s commitment to be one of the global leading and most reliable suppliers of quality diagnostic products and services for personalized healthcare.

Currently, PD-1/PD-L1 monoclonal antibody is one of the most important immunotherapeutic strategies. However, not all cancer patients may benefit from it. Thus, it is an essential clinical requirement to select suitable patients by detecting biomarkers. For the moment, the levels of PD-L1 in tumor tissues is the most clinically recognized predictive bio-markers for the efficacy of anti-PD-1/PD-L1. The global clinical guidelines for non-small cell lung cancer (NSCLC), such as the NCCN guidelines (US), all recommend routine PD-L1 test for patients.
AmoyDx® PD-L1 Expression Detection Kit (IHC) was approved as companion diagnostic assay for Pembrolizumab. An international study shows that E1L3N has good agreement with the mainstream antibodies like, 22C3, 28-8, and SP142[1].
 From 2017 to 2019, NordiQC initiated and consecutively conducted 5 rounds of lung cancer PD-L1 detection quality control, and the detection rate of E1L3N antibody reagent reached 100%[2].
From 2017 to 2019, NordiQC initiated and consecutively conducted 5 rounds of lung cancer PD-L1 detection quality control, and the detection rate of E1L3N antibody reagent reached 100%[2].

In addition, E1L3N antibody is highly consistent with 22C3 at an overall agreement rate up to 95.87%[3], and the good efficacy has verified that the product can better identify patients with NSCLC who are suitable for Pembrolizumab monotherapy.
 More importantly, to get the results within 4 hours, AmoyDx simplifies the preparation steps in the assay. At the same time, a series of optimization carried out based on the mainstream instrument platforms make the PD-L1 expression level can be stably and accurately assessed on different platforms.
More importantly, to get the results within 4 hours, AmoyDx simplifies the preparation steps in the assay. At the same time, a series of optimization carried out based on the mainstream instrument platforms make the PD-L1 expression level can be stably and accurately assessed on different platforms.
About Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685)
Amoy Diagnostics Co., Ltd. is a pioneer and global leading company in the field of molecular diagnostics for precision oncology, focusing on companion diagnostics product development and commercialization. AmoyDx has a portfolio with more than twenty products approved by China’s NMPA, the EU regulatory authorities, Japan’s MHLW, South Korea’s MFDS, etc. Patients in more than 60 countries are benefiting from AmoyDx products. With multiple technological platforms and full capability for companion diagnostics product development and commercialization, AmoyDx has become an important diagnostics partner for many pharmaceutical companies around the globe. For more information, please visit www.amoydiagnostics.com.