

AmoyDx® HRD Focus Panel


Leveraging proprietary HANDLE technology, the Genomic Scar Score (GSS) algorithm, and the AmoyDx NGS Data Analysis System (ANDAS), the HRD Focus assay can be seamlessly integrated into your laboratory workflow.
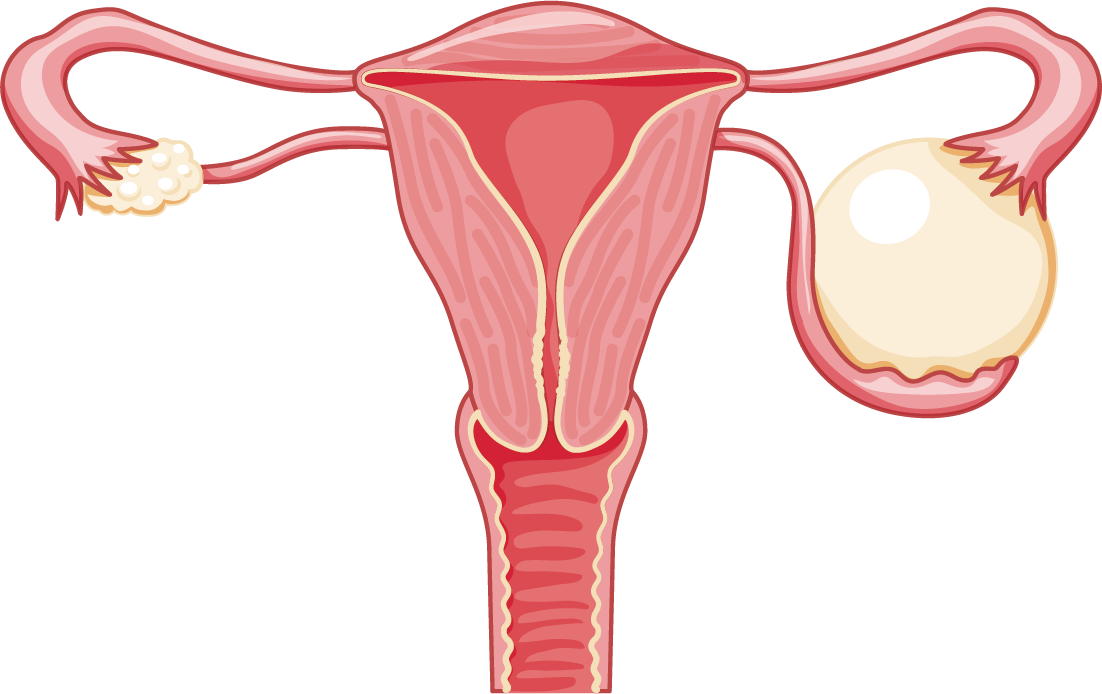
AmoyDx® HRD Complete Panel

A comprehensive homologous recombination deficiency (HRD) test to accelerate PARPi clinical research


AmoyDx® Master Panel

A leading CGP assay that encompasses a wide array of genomic signatures, including HRD, MSI, and TMB, thereby advancing biomarker identification and cancer treatment research across various tumor types.




AmoyDx® BRCA Pro Panel


This panel enables the detection of germline/somatic SNVs/Indels as well as germline large rearrangements (LRs) from either whole blood or FFPE tissue samples.


AmoyDx® HANDLE Classic NGS Panel


The AmoyDx HANDLE Classic Panel encompasses 40 key solid tumor genes, enabling the detection of common mutations to support clinical therapeutic decisions with rapid turnaround time (TAT).



AmoyDx® Essential NGS Panel


The assay is tailored to detect prevalent mutations in 10 genes: EGFR, ALK, ROS1, RET, KRAS, NRAS, PIK3CA, BRAF, HER2, and MET, in patients with non-small cell lung cancer (NSCLC) and colorectal cancer (CRC), using FFPE or Liquid Biopsy samples.


