ONE & ONLY PCR Panel With 167 Variants Across 11 Target Genes in a Single Run
The AmoyDx® Pan Lung Cancer PCR Panel (PLC Panel) is a tissue-based, real-time, qPCR assay for in vitro diagnostics (IVD). The PLC Panel enables qualitative detection of up to 167 variants (85 DNA mutations and 82 RNA fusions) in 11 genes (EGFR, ALK, ROS1, KRAS, BRAF, HER2, RET, MET, NTRK1, NTRK2, and NTRK3), identifying all NCCN recommended biomarkers in a single PCR run. The PLC Panel is intended to assist in identifying clinically relevant biomarkers for patients with non-small cell lung cancer (NSCLC) who may be eligible for approved targeted therapies at minimal cost. The kit is for in vitro diagnostic use and intended to be used by trained professionals in a laboratory environment.
Clinical Performance
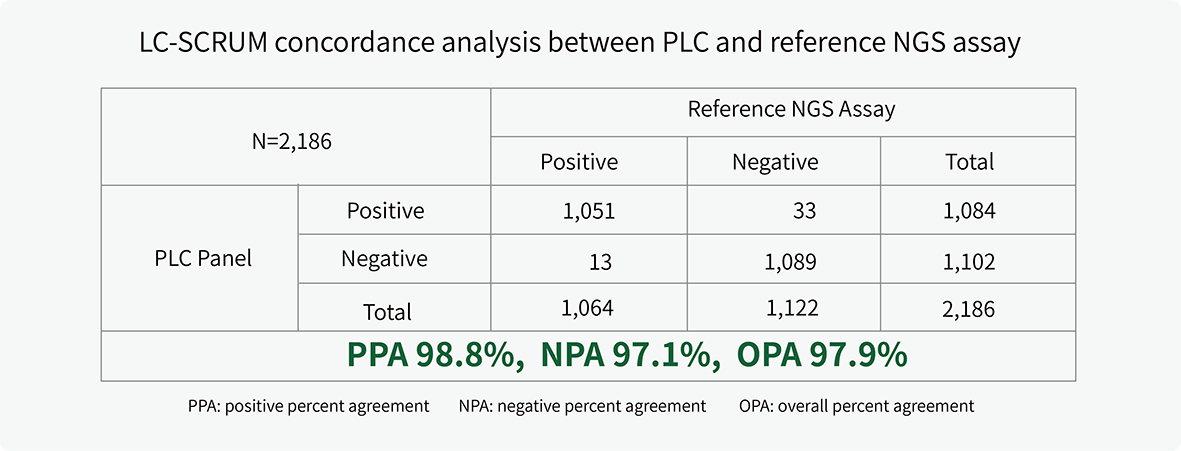
The clinical performance of PLC Panel was validated in a concordance study by LC-SCRUM-Japan.1 Results were highly concordant with the reference NGS assay for alterations (fusion, indels, SNVs) across 11 actionable biomarkers.
1. Matsumoto, S., et. al. Prospective concordance study of a multi-gene PCR assay and NGS for the detection of targetable gene alterations in lung cancer [abstract] in Journal of Thoracic Oncology. 2021 March; 16:3(S690): WCLC; 2020. Abstract P89.06.
Mutations( Hover over each mutational type to highlight genes covered )

EGFR
HER2
KRAS
BRAF
ALK
RET
ROS1
NTRK1
NTRK2
NTRK3
MET

Specifications
Publications


1. Hirotsu, Y., Nakagomi, T., Nagakubo, Y. et al. Simulation analysis of EGFR mutation detection: Oncomine Dx target test and AmoyDx panel impact on lung cancer treatment decisions. Sci Rep 14, 1594 (2024).
2. Takeyasu Y, Yoshida T, Masuda K, et al. Distinct progression and efficacy of first-line osimertinib treatment according to mutation subtypes in metastatic NSCLC harboring EGFR mutations. JTO Clin Res Rep. 2024;5:100636.
3. Sakamoto T, Matsubara T, Takahama T, et al. Biomarker Testing in Patients With Unresectable Advanced or Recurrent Non–Small Cell Lung Cancer. JAMA Netw Open. 2023;6(12):e2347700.
4. Ishioka Y, Tanaka H, Makiguchi T, Fujishima S, Nunomura Y, Sakamoto H, Shiratori T, Taima K and Tasaka S: Predictors of efficacy of anamorelin in patients with non‑small cell lung cancer and cachexia: A retrospective study. Oncol Lett 27: 22, 2024
5. Okimura A, Hirano H, Ito Y, et al. (September 11, 2023) Primary Lung Adenocarcinoma With ALK Gene Rearrangement Mostly Occupied by the Signet-Ring Cell Carcinoma Component: A Case Report. Cureus 15(9): e45068.
6. Pecciarini L, Brunetto E, Grassini G, De Pascali V, Ogliari FR, Talarico A, Marra G, Magliacane G, Redegalli M, Arrigoni G, et al. Gene Fusion Detection in NSCLC Routine Clinical Practice: Targeted-NGS or FISH? Cells. 2023; 12(8):1135.
7. Gu Y, Zhang S, Liang X, Zhao H, Li X, Lu J. Clinical and Pathological Characteristics and Prognosis of Lung Adenocarcinoma With High-Grade Fetal Features: A Retrospective Analysis. International Journal of Surgical Pathology. 2023;0(0).
8. Wang, Lei-Chi et al. Panoramic Tissue Examination That Integrates 3-Dimensional Pathology Imaging and Gene Mutation: Potential Utility in Non–Small Cell Lung Cancer. Laboratory Investigation, Volume 103, Issue 9, 100195.
9. S. Matsumoto, T. Seto, C. Svedman, T. Kato, K. Nishino, N. Furuya, Y. Ohe, R. Toyozawa, M. Kodani, M. Shingyoji, Y. Shibata, K. Nosaki, H. Izumi, Y. Zenke, Y. Murata, K. Yoh, K. Goto. Clinical concordance of Pan Lung Cancer PCR Panel covering 167 actionable variants across 11 genes and other validated assays in the LC-SCRUM-Asia registry. JTO Clinical and Research Reports, 2025, 100920.
10. Wan-Shan Li et al. Implementation of Real-World Diagnostic Strategies in Taiwan for the Identification of Targetable Oncogenic Driver Alterations in Non–Small Cell Lung Cancer. JCO Glob Oncol 11, e2500144(2025).






