The 10th-anniversary conference of the Asian Lung Cancer Genome Screening Project (LC-SCRUM) was held at Tokyo, Japan on the 23rd of December 2023.
Dr. Limou Zheng, Founder of Amoy Diagnostics, and Mr. Frank Zheng, Chief Operating Officer, were honored guests and keynote speakers at the event. Initiated in 2013 by the National Cancer Center of Japan, LC-SCRUM is a pioneering Asian cancer gene screening project for precision oncology. It encompasses over 200 hospitals, pharmaceutical companies, and diagnostic firms across Japan, significantly advancing clinical trials and the approval of innovative targeted lung cancer drugs in the country.
Amoy Diagnostics, the sole Chinese diagnostic company selected for this project, has made notable contributions with its innovative products. The AmoyDx® Pan Lung Cancer PCR Panel (PLC Panel), utilizing Multi-ARMS rapid screening technology, and the comprehensive AmoyDx® Master Panel have both received widespread acclaim for their exceptional performance in clinical and research settings globally.
Professor Koichi Goto, the lead of the LC-SCRUM project, provided an insightful overview of the project's ten-year journey. He emphasized the collaborative efforts of various pharmaceutical and diagnostic companies, highlighting the significant advancements in lung cancer clinical practices across the Asia-Pacific region. Professor Goto specifically commended the role of Amoy Diagnostics' products, such as the PLC Panel. This single-test solution, capable of detecting nine critical driver genes in lung cancer, offers a swift testing process, enabling rapid and accurate identification of patients suitable for targeted therapies and reducing the waiting time for treatment.
Expressing his gratitude, Professor Goto acknowledged Amoy Diagnostics' pivotal role in advancing the precision diagnosis and treatment of lung cancer. Dr. Limou Zheng, in his address, reflected on Amoy Diagnostics' journey with LC-SCRUM: "As a primary collaborative partner, we have closely observed the development and achievements of LC-SCRUM over the past decade. From our initial companion diagnostic clinical research with Pfizer on Ros1 testing for Crizotinib in 2013 to conducting companion diagnostic clinical studies for 14 pharmaceutical companies targeting nine driver genes in lung cancer, and the subsequent approval and inclusion of our products in the Japanese medical insurance system, Amoy Diagnostics has consistently been recognized by regulatory authorities and oncology experts in Japan for our commitment to medical excellence. We take pride in providing accurate and reliable diagnostic solutions to patients worldwide!”
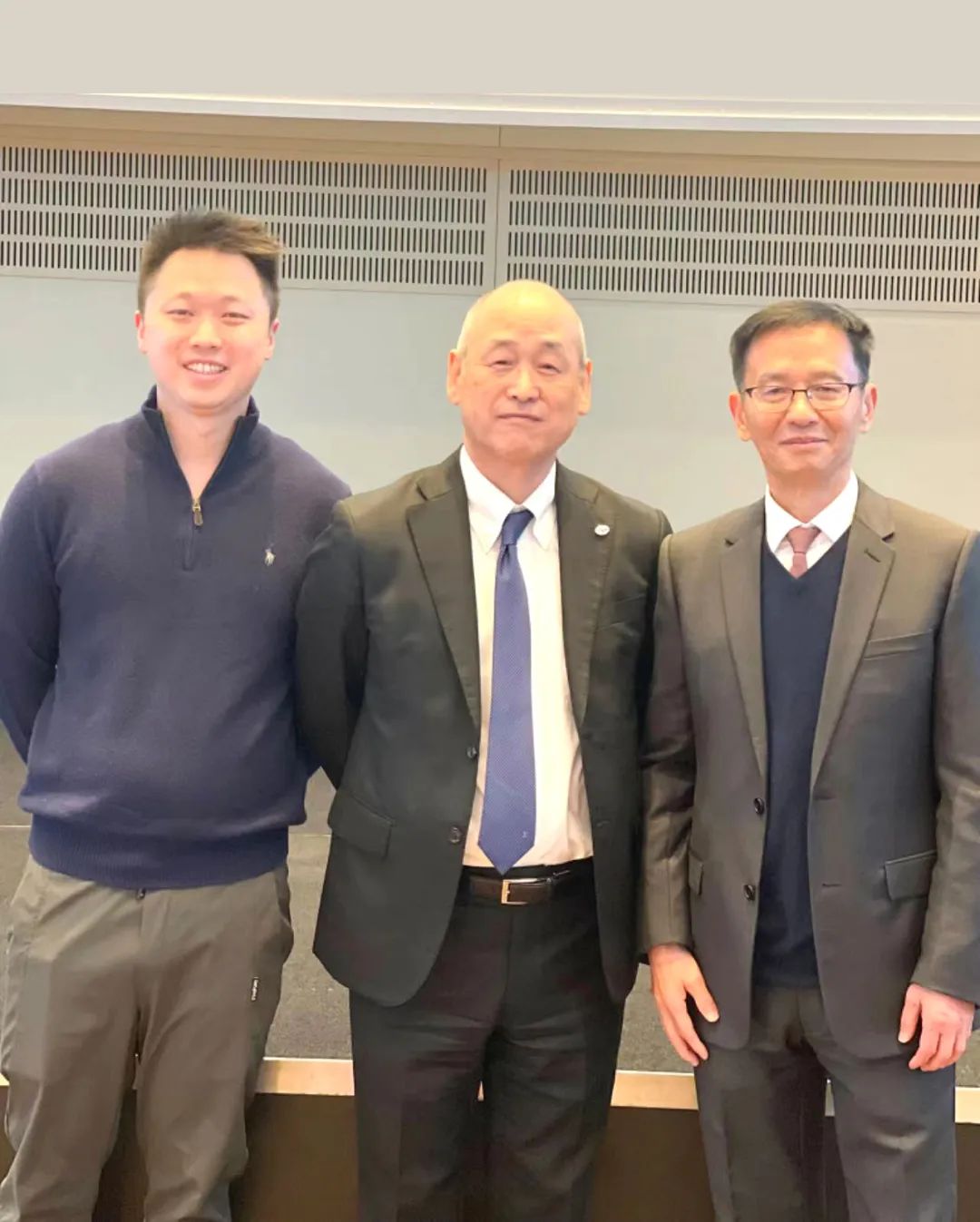
Dr. Limou Zheng, Founder of Amoy Diagnostics, and Mr. Frank Zheng, Chief Operating Officer, were honored guests and keynote speakers at the event. Initiated in 2013 by the National Cancer Center of Japan, LC-SCRUM is a pioneering Asian cancer gene screening project for precision oncology. It encompasses over 200 hospitals, pharmaceutical companies, and diagnostic firms across Japan, significantly advancing clinical trials and the approval of innovative targeted lung cancer drugs in the country.
Amoy Diagnostics, the sole Chinese diagnostic company selected for this project, has made notable contributions with its innovative products. The AmoyDx® Pan Lung Cancer PCR Panel (PLC Panel), utilizing Multi-ARMS rapid screening technology, and the comprehensive AmoyDx® Master Panel have both received widespread acclaim for their exceptional performance in clinical and research settings globally.
Professor Koichi Goto, the lead of the LC-SCRUM project, provided an insightful overview of the project's ten-year journey. He emphasized the collaborative efforts of various pharmaceutical and diagnostic companies, highlighting the significant advancements in lung cancer clinical practices across the Asia-Pacific region. Professor Goto specifically commended the role of Amoy Diagnostics' products, such as the PLC Panel. This single-test solution, capable of detecting nine critical driver genes in lung cancer, offers a swift testing process, enabling rapid and accurate identification of patients suitable for targeted therapies and reducing the waiting time for treatment.
Expressing his gratitude, Professor Goto acknowledged Amoy Diagnostics' pivotal role in advancing the precision diagnosis and treatment of lung cancer. Dr. Limou Zheng, in his address, reflected on Amoy Diagnostics' journey with LC-SCRUM: "As a primary collaborative partner, we have closely observed the development and achievements of LC-SCRUM over the past decade. From our initial companion diagnostic clinical research with Pfizer on Ros1 testing for Crizotinib in 2013 to conducting companion diagnostic clinical studies for 14 pharmaceutical companies targeting nine driver genes in lung cancer, and the subsequent approval and inclusion of our products in the Japanese medical insurance system, Amoy Diagnostics has consistently been recognized by regulatory authorities and oncology experts in Japan for our commitment to medical excellence. We take pride in providing accurate and reliable diagnostic solutions to patients worldwide!”

Highlights of China International Import Expo (CIIE) 2023 - Amoy Diagnostics Announced Multiple Collaborations with AstraZeneca and Pfizer
Next
Subscribe
to our
newsletter
