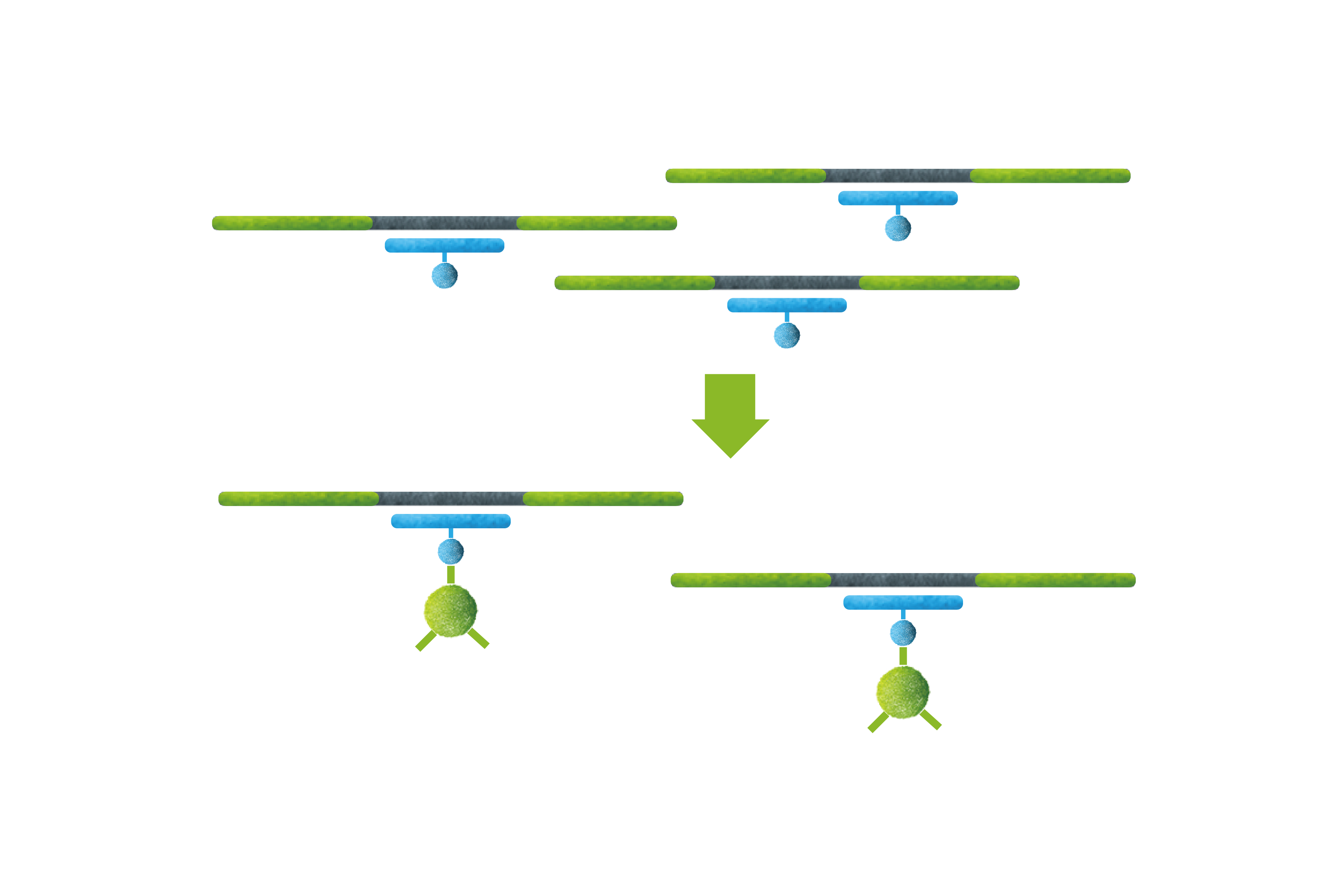
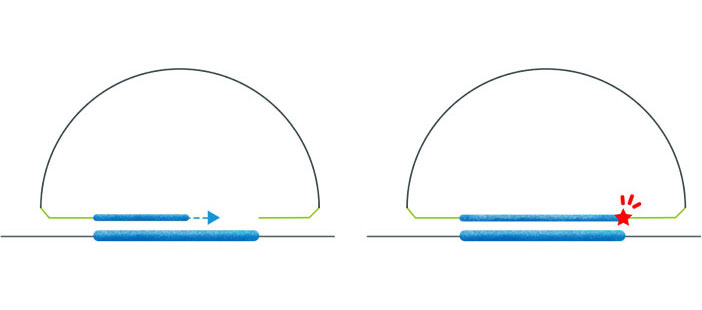
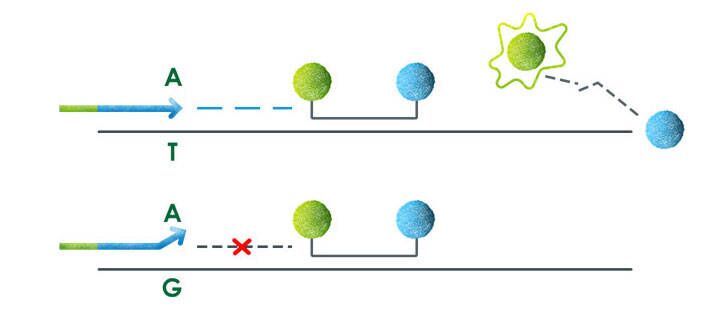
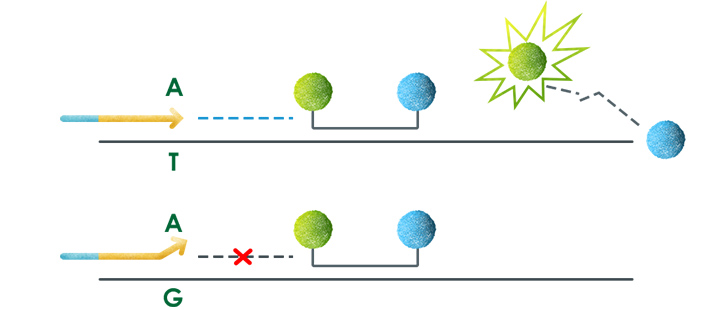
Welcome to AmoyDx

A visionary diagnostic company dedicated to transforming the landscape of cancer care. 'Breaking Barriers to Precision Oncology' reflects our commitment to revolutionizing cancer diagnosis and treatment.
We are driven by four fundamental values: Innovation in Care, Accessibility and Equity, Collaboration and Partnership, and Integrity and Trust.
These values guide our pursuit of developing groundbreaking diagnostic tools and fostering a more equitable, collaborative, and trustworthy approach to oncology.
Join us as we pave the way to a future where precision cancer care is a reality for every patient, everywhere.