- 1Department of Thoracic Oncology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
- 2Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- 3Department of Thoracic Surgery, Kitakyushu Municipal Medical Center, Fukuoka, Japan
Osimertinib is a standard therapy for the treatment of advanced non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor gene (EGFR) mutations, but most patients with EGFR-mutant NSCLC develop secondary resistance to osimertinib. Mesenchymal-epithelial transition gene (MET) alterations and oncogene fusions have been identified as the most common mechanisms of resistance to osimertinib. However, MET exon 14 skipping mutation (METex14del) as an acquired resistance to osimertinib has rarely been reported. A non-smoking 76-year-old woman was diagnosed with lung adenocarcinoma in the right lower lobe (cT2bN2M1c [pulmonary and bone metastases], cStage IVB). The primary tumor was submitted to cobas® EGFR Mutation Test v2 (Roche Diagnostics Ltd.), next generation sequencing (Oncomine Comprehensive Assay v3; Thermo Fisher Scientific), the AmoyDx® Essential NGS panel (Amoy Diagnostics, Xiamen, China), all of which were positive for EGFR L858R and de novo T790M. We administered daily osimertinib (80 mg/day), and achieved a partial response. However, after 14.0 months, computed tomography showed progression of the primary tumor and lung metastases. Re-biopsy of the primary tumor was conducted, and the specimen was submitted to Archer®MET companion diagnostic for detection of METex14del. Although the primary tumor was negative for METex14del, the re-biopsy specimen was positive for METex14del. We validated that the biopsy specimen of the primary tumor at diagnosis before osimertinib administration was negative for METex14del using local reverse transcription PCR. We administered daily tepotinib (500 mg/day) to the patient as a further-line treatment, and achieved a partial response (tumor shrinkage rate: 34.5%) after 2.0 months, who responded to tepotinib therapy for 8.0 months. We described a patient with lung adenocarcinoma harboring METex14del as a potential acquired resistance to osimertinib, who responded to subsequent tepotinib therapy. Re-biopsy and re-analysis of genetic profiles should be considered in NSCLC patients who develop osimertinib resistance.
Introduction
Epidermal growth factor receptor gene (EGFR)-tyrosine kinase inhibitors (TKIs) are approved by the United States Food and Drug Administration for the treatment of advanced non-small cell lung cancer (NSCLC) harboring EGFR mutations (1). Osimertinib (Tagrisso™, [AZD9291] AstraZeneca, Cambridge, UK), a third-generation, irreversible, EGFR-TKI, is an approved therapy (80 mg, once-daily) for treatment of patients with metastatic EGFR-mutated NSCLC and those with T790M-positive NSCLC after disease progression on EGFR-TKIs (1, 2). However, despite very high objective response rates, most patients with EGFR-mutant NSCLC develop secondary resistance to EGFR-TKIs. Secondary resistance can be broadly classified into EGFR-dependent and independent mechanisms (3). Mesenchymal-epithelial transition gene (MET) alterations, EGFR C797X, small cell lung cancer transformation, and oncogene fusions have previously been identified as the most common mechanisms of resistance to EGFR-TKIs (3).
MET exon 14 skipping mutation (METex14del), a splice-site oncogenic mutation, is found in 2%–3% of NSCLC patients (4). Such patients respond well to MET-tyrosine kinase inhibitors, including tepotinib (5). Tepotinib is a once-daily oral type Ib (highly selective) MET inhibitor, blocking ATP binding to prevent phosphorylation and activation of the MET receptor. Tepotinib has shown promising clinical activity in patients with NSCLC harboring METex14del, with the response rate of 46% (95% confidence interval, 36 to 57) and a median duration of response of 11.1 months (5). Although MET amplification has already been reported from the viewpoint of secondary resistance to EGFR-TKIs, METex14del has rarely been documented (3, 6). We herein report a rare case of a patient with lung adenocarcinoma harboring the METex14del mutation as a potential acquired resistance to osimertinib, who responded to subsequent tepotinib therapy.
Case report
A non-smoking 76-year-old woman was diagnosed with pulmonary nodules following a cancer screening. Several radiological examinations and bronchial biopsy led to a diagnosis of primary lung adenocarcinoma in the right lower lobe (cT2bN2M1c [pulmonary and bone metastases], cStage IVB according to the 8th Edition of TNM in Lung Cancer). The primary tumor was submitted to cobas® EGFR Mutation Test v2 (Roche Diagnostics Ltd.), next generation sequencing (Oncomine Comprehensive Assay v3; Thermo Fisher Scientific), and the AmoyDx® Essential NGS panel (Amoy Diagnostics, Xiamen, China), all of which were positive for EGFR L858R and de novo T790M. We administered daily osimertinib (80 mg/day), and achieved a partial response. However, after 14.0 months, chest computed tomography (CT) showed progression of the primary tumor and lung metastases.
As a second-line therapy, pemetrexed (500 mg/m2) had been administered for 6.7 months. The CT scan subsequently showed right femoral bone metastasis, and palliative radiation therapy (30 Gy/10 Fr) was administered because of the pain. Re-biopsy of the primary tumor was conducted, and the specimen was submitted to Archer®MET companion diagnostic for detection of METex14del, which is a companion diagnostic for tepotinib. This was positive for METex14del. The re-biopsy specimen was positive for EGFR L858R and de novo T790M, although the biopsy specimen of the primary tumor before osimertinib administration was negative for METex14del using local reverse transcription PCR (Figure 1).
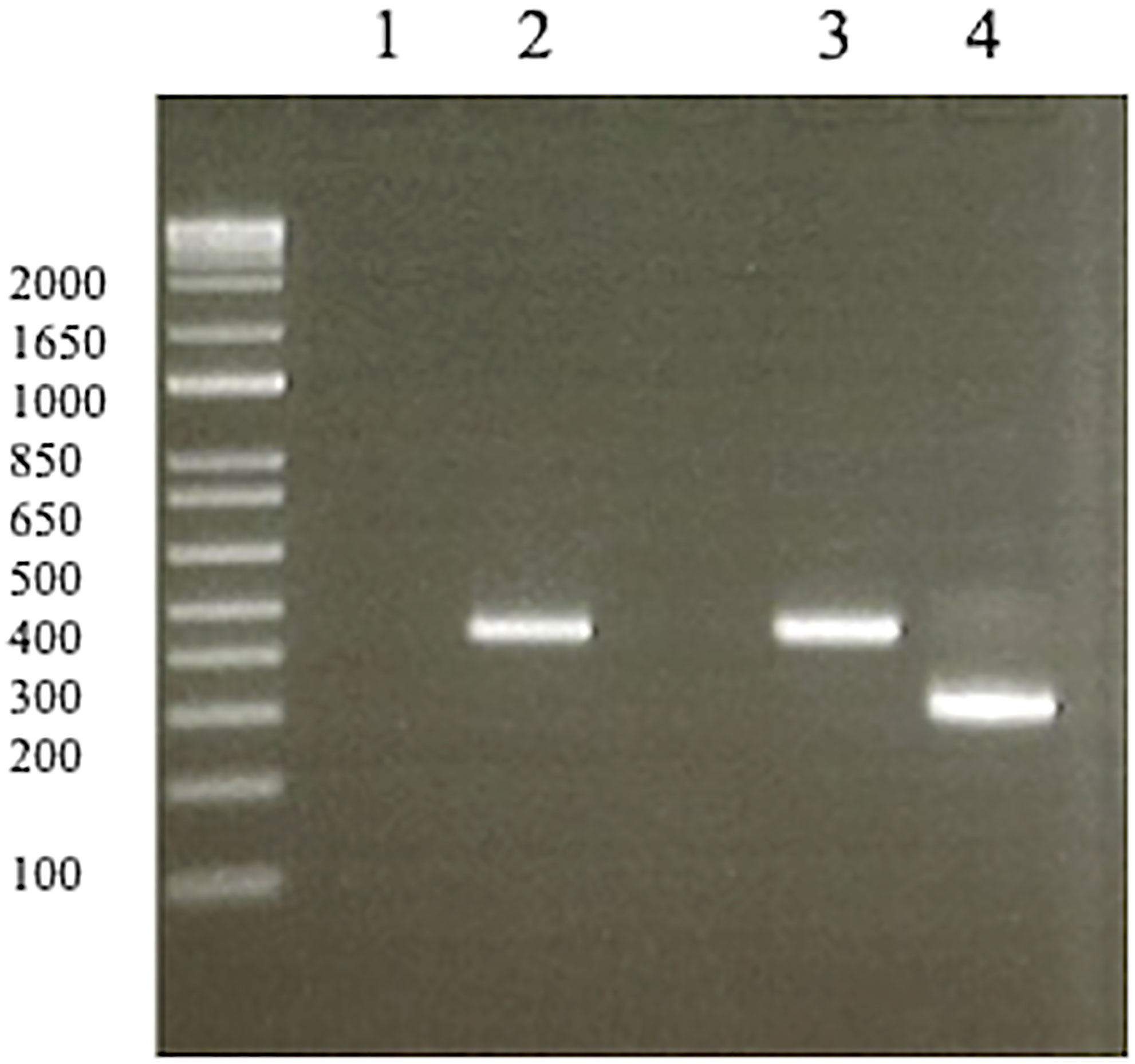
Figure 1 Reverse transcription PCR of negative/positive controls (1, 3, 4) and biopsy specimen before osimertinib administration from the patient (2). (1) No template (negative control). (2) The biopsy specimen before osimertinib administration was negative for METex14del. (3) METex14del wild type sample (negative control). (4) METex14del positive sample (positive control). MET, mesenchymal-epithelial transition gene.
We administered daily tepotinib (500 mg/day) to the patient as a further-line therapy, and achieved a partial response (tumor shrinkage rate: 34.5% in diameter) after 2.0 months (Figures 2A, B). Although the tumor had been responded to tepotinib therapy for 8.0 months, CT and positron emission tomography revealed left femoral bone metastasis as a new lesion. After subsequent therapies (nab-paclitaxel [100 mg/m2 on days 1, 8, 15] for 1.3 months and S-1 [100 mg/body on days 1-14] for 1.0 month), the patient died because of disease progression 32.3 months after administration of first-line osimertinib therapy.
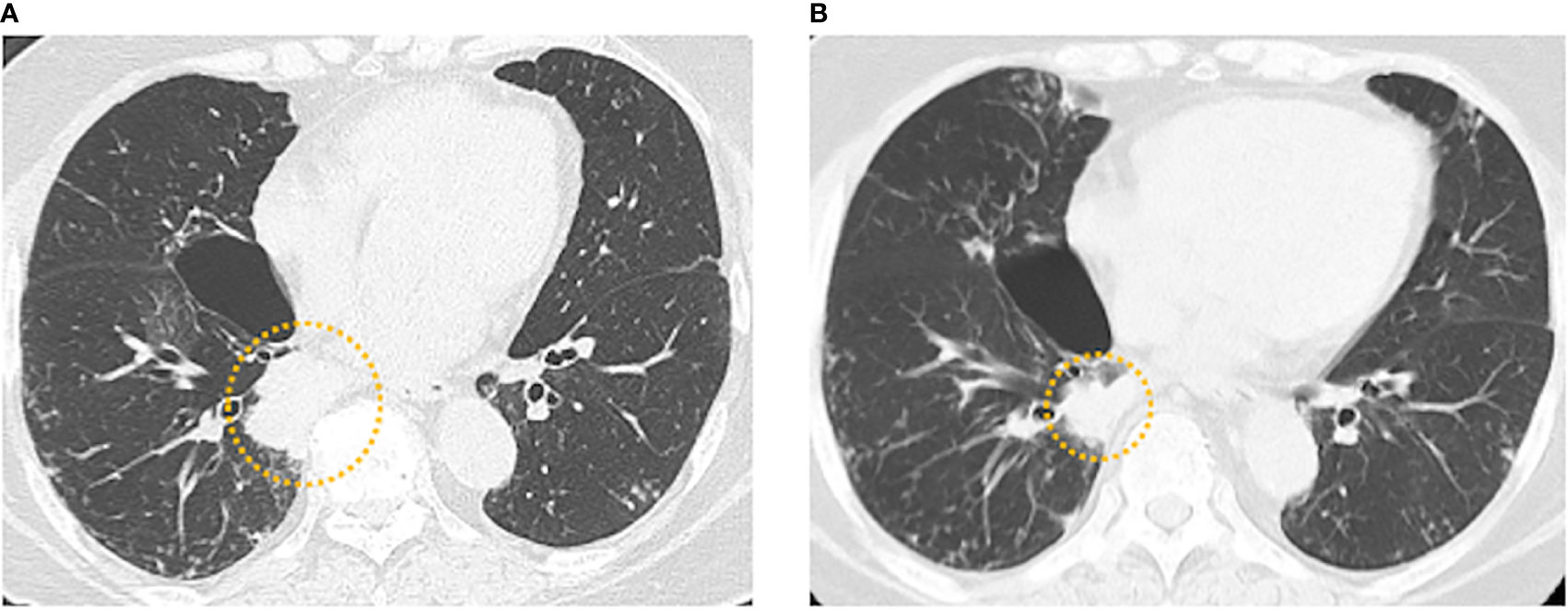
Figure 2 (A) Baseline chest computed tomography (CT) before tepotinib administration showing the tumor in the right lower lobe. (B) Chest CT 2.0 months after tepotinib administration showing a partial response (tumor shrinkage rate: 34.5% in diameter).
Discussion
We herein report a rare case of a patient with lung adenocarcinoma harboring METex14del following resistance to osimertinib, who responded to subsequent tepotinib therapy. We have identified two possible hypotheses that could explain this phenomenon. First, METex14del was not present before osimertinib therapy, but was induced by osimertinib as an acquired resistance. Second, METex14del was present at very low copy numbers in the lung adenocarcinoma, and was not detected in the original biopsy because of tumor heterogeneity, suggesting the coexistence of EGFR and METex14del mutations.
With regard to METex14del as an acquired resistance, a few studies have reported similar findings (6, 7). Pinquie F et al. reported a case of treatment with crizotinib to overcome resistance to osimertinib in an EGFR-mutant NSCLC patient harboring an acquired METex14del. In that report, the CT-scan after 4 months crizotinib of monotherapy showed a complete response of lung metastases, progression of liver metastases, and reappearance of pleural nodules. Thus, the clinical activity of crizotinib was heterogeneous, and crizotinib monotherapy could not confirm a partial response. In our report, a partial response was achieved with tepotinib therapy, and the duration of response was 8.0 months. The discrepancy in efficacy may be due to the difference in types of MET-TKIs. Crizotinib is a type Ia inhibitor, blocking ATP binding to prevent phosphorylation/activation of the receptor, whereas tepotinib is a type Ib inhibitor, blocking MET more specifically than type Ia inhibitors (8). According to preclinical work by Suzuwa et al. (7), cell viability assays using an EGFR-mutant NSCLC cell model transfected with a lentiviral vector expressing METex14del revealed that METex14del reduced sensitivity to osimertinib, and METex14del expression correlated with upregulation of phosphorylated EGFR. These results indicated that METex14del induces resistance to osimertinib in EGFR-mutant NSCLC cells. Suzuwa et al. also reported that crizotinib monotherapy only inhibited METex14del phosphorylation in EGFR-mutant and METex14del-transfected NSCLC cell, and did not block phosphorylation of EGFR, AKT, and ERK, which suggests that EGFR is still signaling (7). Accordingly, a combination therapy of osimertinib and MET inhibitors was effective against METex14del-induced drug resistance in EGFR-mutant NSCLC cells. The treatment of patients with two driver oncogene mutations is thought to require the inhibition of both regulatory pathways, which is being investigated in ongoing clinical trials (9, 10). Regarding the coexistence of EGFR and METex14del mutations, a previous study reported concomitant EGFR and METex14del frequencies of 0.2% (three out of 1,590 cases in a Chinese cohort) (11). There is a possibility that METex14del was present at very low copy numbers in the original biopsy specimen, but we confirmed that the biopsy specimen of the primary tumor before osimertinib administration was negative for METex14del using local reverse transcription PCR. In any case, re-biopsy and re-analysis of genetic profiles in NSCLC should be considered after osimertinib resistance.
In our patient, the duration of the response to tepotinib (8.0 months) was slightly shorter than the median duration of response reported in a previous clinical trial (11.1 months) (5). This may be because de novo and acquired METex14del differ in their response to tepotinib, and/or because EGFR and METex14del co-mutations require both EGFR- and MET-TKI therapies (12). Nevertheless, the duration of response to tepotinib is expected to be longer than that of chemotherapy in the treatment of lung adenocarcinoma with EGFR and METex14del mutations. Therefore, we recommend that re-biopsy and re-analysis of genetic profiles should be considered in NSCLC patients who have developed osimertinib resistance.
Conclusion
We report a rare case of a patient with lung adenocarcinoma harboring METex14del as a potential acquired resistance to osimertinib, who responded to tepotinib therapy. Re-biopsy and re-analysis of genetic profiles in NSCLC should be considered in cases of osimertinib resistance.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review boards of National Hospital Organization Kyushu Cancer Center (IRB No. 2019-45). Written informed consent for publication was obtained from the legally authorized representative of the patient.
Author contributions
ST treated the patient and wrote the manuscript. TS significantly contributed to all of the ideas and methods. MY and TO assisted and supervised all of the analyses conducted in this study. FK assisted in drafting the manuscript. TF and FS treated the patient. KI and RT supervised the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgment
We thank Sarah Williams, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-Small-Cell lung cancer. N Engl J Med (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
2. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med (2017) 376:629–40. doi: 10.1056/NEJMoa1612674
3. Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: A review. J Clin Oncol (2020) 38(25):2926–36. doi: 10.1200/JCO.19.03123
4. Huang C, Zou Q, Liu H, Qiu B, Li Q, Lin Y, et al. Management of non-small cell lung cancer patients with MET exon 14 skipping mutations. Curr Treat Options Oncol (2020) 21:33. doi: 10.1007/s11864-020-0723-5
5. Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non-Small-Cell lung cancer with MET exon 14 skipping mutations. N Engl J Med (2020) 383:931–43. doi: 10.1056/NEJMoa2004407
6. Pinquie F, Cortot AB, Chevalier L-M, Morel A, Sandrini J, Guguen C, et al. A case report of successful treatment with crizotinib to overcome resistance to osimertinib in an EGFR mutated non–Small-Cell lung cancer patient harboring an acquired MET exon 14 mutation. Clin Lung Cancer (2022) 23:e131–4. doi: 10.1016/j.cllc.2021.06.002
7. Suzawa K, Offin M, Schoenfeld AJ, Plodkowski AJ, Odintsov I, Lu D, et al. Acquired MET exon 14 alteration drives secondary resistance to epidermal growth factor receptor tyrosine kinase inhibitor in EGFR-mutated lung cancer. JCO Precis Oncol 3 (2019), PO.19.00011. doi: 10.1200/PO.19.00011
8. Brazel D, Zhang S, Nagasaka M. Spotlight on tepotinib and capmatinib for non-small cell lung cancer with MET exon 14 skipping mutation. Lung Cancer (Auckl) (2022) 13:33–45. doi: 10.2147/LCTT.S360574
9. Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H, Kim SW, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol (2020) 21:373–86. doi: 10.1016/S1470-2045(19)30785-5
10. Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: a multi-arm, phase ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol (2020) 31:507–16. doi: 10.1016/j.annonc.2020.01.013
11. Li W-F, Kang J, Zhang X-C, Jian S, Chen H, Wang Z, et al. Coexistence of MET exon 14 mutations with EGFR mutations in non-small cell lung cancer. J Clin Oncol (2017) 35:e20636–6. doi: 10.1200/JCO.2017.35.15_suppl.e20636
Keywords: lung adenocarcinoma, METex14del, tepotinib, osimertinib, resistance
Citation: Takamori S, Seto T, Yamaguchi M, Kinoshita F, Fujishita T, Ito K, Toyozawa R, Shoji F and Okamoto T (2022) Case report: Success of tepotinib therapy in overcoming resistance to osimertinib in a patient with EGFR-mutant lung adenocarcinoma with a potential acquired MET exon 14 skipping mutation. Front. Oncol. 12:965741. doi: 10.3389/fonc.2022.965741
Received: 10 June 2022; Accepted: 30 September 2022;
Published: 13 October 2022.
Edited by:
Luka Brcic, Medical University of Graz, AustriaReviewed by:
Sebahat Ocak, CHU UCL Namur Site Godinne, BelgiumZhuo-Xun Wu, St. John’s University, United States
Copyright © 2022 Takamori, Seto, Yamaguchi, Kinoshita, Fujishita, Ito, Toyozawa, Shoji and Okamoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takashi Seto, setocruise@gmail.com
 Shinkichi Takamori
Shinkichi Takamori Takashi Seto
Takashi Seto Masafumi Yamaguchi
Masafumi Yamaguchi Fumihiko Kinoshita1
Fumihiko Kinoshita1